Discussion
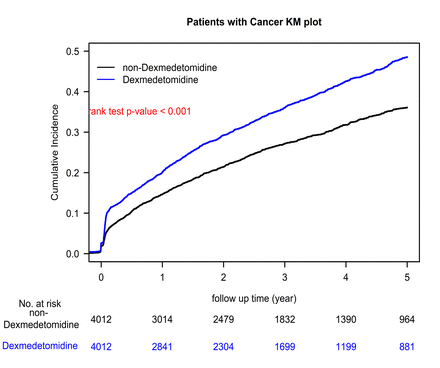
This is the first clinical study to examine the association between perioperative DEX use and oncological outcomes in OCSCC. Using a propensity score-matched design to approximate an RCT, we found that perioperative DEX was significantly associated with increased risks of LRR and DM, though not with OS. These findings highlight a potential adverse impact of DEX in standard curative treatment for OCSCC.
DEX is a highly selective α2-adrenergic receptor agonist, with an α2/α1 ratio of 1620.37 Its use in surgeries significantly increases the number of same-day discharges, reduces postoperative analgesic consumption and mitigates side effects such as nausea, vomiting and bleeding, making it widely used in surgical patients.38 39 Previous clinical studies examining the relationship between DEX and postoperative cancer recurrence are limited. Activation of α2-adrenergic receptors has been reported to lead to breast cancer cell relapse or metastasis, as demonstrated in several preclinical studies involving both cell lines and rodent models.17 40 41 Preclinical studies have shown that DEX may promote metastasis, as evidenced by increased tumour cell retention in models of mammary adenocarcinoma, Lewis lung carcinoma and colon adenocarcinoma.18 Clinical data remain limited. In a retrospective PSM study, Cata et al found that intraoperative DEX use in non-small cell lung cancer patients was associated with shorter OS, possibly due to enhanced minimal residual disease progression.13 No RCTs have yet evaluated DEX’s oncological effects. Our study is the first to use PSM to examine DEX in OCSCC patients undergoing curative surgery, revealing its association with increased risks of postoperative LRR and DM. The absence of significant differences in OS or CSS may relate to the predominance of advanced-stage disease (stage IV >50%), where cancer stage may exert a stronger prognostic influence than DEX exposure. Further analyses in early-stage OCSCC patients are planned to clarify the survival impact of perioperative DEX.
The mechanisms by which DEX promotes postoperative LRR and DM remain unclear. Studies suggest two key pathways: direct activation of α2-adrenergic receptors on cancer cells and indirect activation via host stromal cells.16 17 EX also acts on imidazoline receptors, increasing hypoxia-inducible factors and vascular endothelial growth factor (VEGF), which facilitate tumour growth.42 43 Additionally, DEX may suppress immune function. Inada et al reported that long-term subhypnotic DEX use reduced cytotoxic lymphocyte activity and enhanced tumour growth in lymphoma-bearing animals.44 These mechanisms may explain the elevated LRR and DM risks observed in our DEX-treated OCSCC cohort (tables 1 and 2). Although LRR and DM represent distinct modes of oncological failure, our study observed significant associations between perioperative DEX use and increased risks for both outcomes. These findings suggest that DEX may exert both local and systemic effects conducive to tumour recurrence and metastasis. Further mechanistic studies are warranted to determine whether these observations reflect shared biological pathways, such as immune suppression or angiogenic activation, or represent independent DEX-related processes influencing local control and systemic spread, respectively.
These findings also underscore the biological plausibility of how a single perioperative event—such as DEX administration—can result in long-term oncological consequences.45–48 The perioperative period is increasingly recognised as a critical window for cancer progression and metastasis.45–48 Surgery and associated stress can promote tumour cell dissemination, suppress antitumour immunity and enhance the metastatic potential of residual disease.47 Metabolic, inflammatory and neuroendocrine changes during this period—exacerbated by perioperative interventions such as anaesthesia, transfusion and hypothermia—may all contribute to cancer evolution.45 46 Cancer cells may exploit this vulnerable physiological state to remodel the tumour microenvironment and escape immune detection, leading to early micrometastatic seeding or future recurrence.45 48 In this context, DEX has been shown in preclinical studies to promote cancer cell proliferation and invasion through α2-adrenergic receptor-mediated pathways and to act via imidazoline receptors to upregulate hypoxia-inducible factor-1α and VEGF, thereby facilitating angiogenesis and tumour progression.49–52 Furthermore, DEX suppresses antitumour immunity by impairing natural killer (NK) cell activity and cytotoxic T lymphocyte function, which could permit early metastatic outgrowth.53–56 Such mechanisms support our clinical observation that perioperative DEX use is associated with increased risks of LRR and DM manifesting years after surgery, particularly among immunocompromised or high-risk OCSCC patients.45–48 53–56
Based on our findings, which are derived from retrospective observational data, clinicians should exercise caution and consider the potential oncological risks associated with perioperative DEX use. While these results raise important clinical concerns, further validation through prospective studies or randomised trials is needed before formal changes in treatment strategies can be recommended. Our findings indicate a significant association between perioperative DEX use and increased risks of LRR and DM in OCSCC patients. Clinicians should exercise heightened caution when considering the use of DEX in the perioperative period for these patients. Given the potential adverse effects of DEX on oncological outcomes, careful patient selection is crucial. Future research should focus on early-stage OCSCC patients to better understand the impact of perioperative DEX on survival outcomes, helping delineate the subset of patients who may safely benefit from DEX without an increased risk of recurrence or metastasis. Additionally, future research should prioritise the development and validation of alternative perioperative sedation protocols that safeguard oncological outcomes in OCSCC patients. Further studies are needed to elucidate the mechanisms by which DEX promotes LRR and DM, as this could inform targeted strategies to mitigate its adverse effects. Clinical guidelines should consider these findings, and educational programmes should raise awareness among providers about the potential risks of perioperative DEX in OCSCC. Incorporating these insights into practice may help optimise perioperative care and improve long-term outcomes for patients, minimising unintended harm to survival or quality of life.
In Taiwan, OCSCC predominantly originates from buccal mucosa, largely due to the high prevalence of betel nut chewing. This carcinogenic habit contributes to extensive field cancerisation and is strongly associated with higher rates of LRR, as reported in previous Taiwanese cohorts.7–9 In our study, the observed association between perioperative DEX use and increased LRR should be interpreted with caution, especially in regions with high betel nut consumption. Although these findings may not be directly generalisable to Western populations, they underscore the importance of individualised perioperative management strategies in areas where betel nut chewing is endemic. Our study found that perioperative DEX use is significantly associated with increased LRR in OCSCC, warranting caution in clinical application. LRR is the most common failure pattern in betel nut-associated OCSCC.7–9 While generalising these findings to regions with lower betel nut use may be limited, the high recurrence risk observed reinforces the need for careful evaluation of perioperative DEX in high-prevalence areas. Moreover, OCSCC’s high metastatic potential may involve immune evasion mechanisms.44 Despite robust screening in Taiwan, most OCSCC patients are diagnosed at stage IV (online supplemental table 1), diminishing the survival impact of DEX (table 1; online supplemental figure 2). Therefore, perioperative DEX should be used judiciously, and future studies should focus on early-stage patients to better assess its survival effects.