
2022 Highlights – EDQM annual report now available
The European Directorate for the Quality of Medicines & HealthCare (EDQM) has published its 2022 annual report, providing a comprehensive overview of its activities and achievements, and putting select data on the present state of EDQM/Council of Europe public health initiatives at your fingertips. The EDQM continued to adapt to a changing environment in 2022, looking resolutely forward while responding
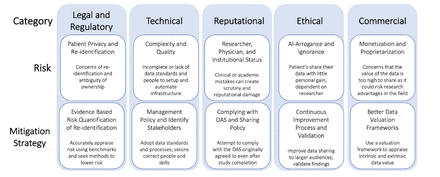
Delivering on NIH data sharing requirements: avoiding Open Data in Appearance Only
AI arrogance and ignorance The current system means that the risk for sharing one’s data is high, with little personal gain. Despite the fact these risks are real to the institution, the failure to disclose data does not eliminate the risk; it merely transfers the risk from the institution to the patients being treated based on the research. Thus, those
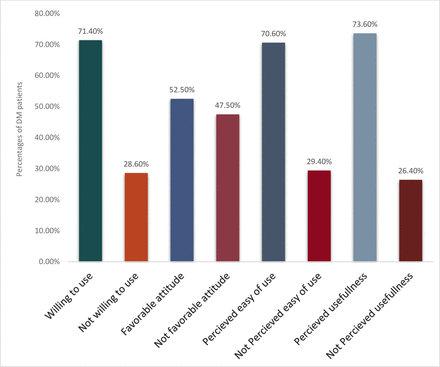
Willingness of diabetes mellitus patients to use mHealth applications and its associated factors for self-care management in a low-income country: an input for digital health implementation
Discussion The purpose of this study was to assess willingness of patients with DM to use mobile health applications and associated factors for self-care management. The result showed that willingness to use mHealth applications among patients with DM in the Oromia region was high (71.4%, 95% CI (66.8% to 75.9%)). This result was in line with the willingness to use mHealth
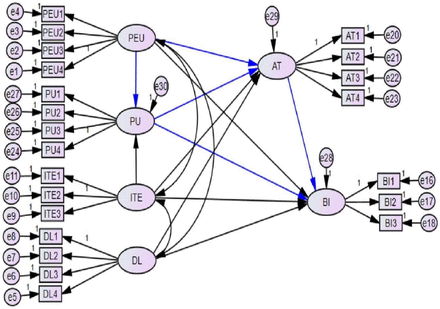
Predicting healthcare professionals’ acceptance towards electronic personal health record systems in a resource-limited setting: using modified technology acceptance model
Introduction Over the past decades, a variety of eHealth technologies have been accessible as nations have implemented eHealth efforts to support the objectives for health education and person-centred care.1 Adoption of personal health records (PHRs) has been linked to numerous advantages, including improved patient–provider relationships, patient engagement improvements, better medication adherence, good health outcomes (such as blood pressure and glycaemic
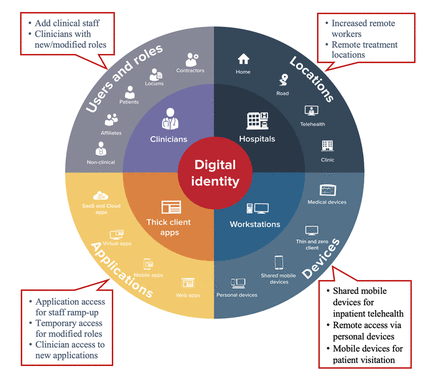
COVID-19 surge readiness: use cases demonstrating how hospitals leveraged digital identity access management for infection control and pandemic response
Results Table 1 summarises the hospital facility value and functional focus of each of the eight use cases reported. Table 1 View inline•Open as popup Use cases of IAM technology deployed in hospital COVID-19 response by value and functional focus SSO enabled clinicians to attest being symptom free at shift start During an outbreak of a highly transmissible pathogen such
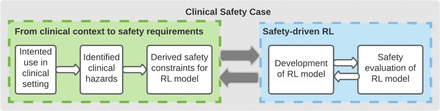
Assuring the safety of AI-based clinical decision support systems: a case study of the AI Clinician for sepsis treatment
Discussion Systematic safety assessment of AI-based clinical decision support systems is poorly codified, especially in applications where the definition of effective and safe decisions is challenging. In this study, we applied best practice in safety assurance to a complex AI system and proposed a safety-driven approach to identify regions of the action space potentially associated with preventable harm. We showed

EDQM OCCL study reports low compliance of “kids’ cosmetics”
A study by the European Network of Official Cosmetics Control Laboratories (OCCLs) indicates that “kids’ cosmetics” continue to fail to comply with European quality and safety regulations, finding that 25% of samples were non-compliant with legislative requirements. Decorative cosmetics, including temporary hair colour products (54% of the samples), nail varnishes (39%), body and face paints (25%), eye products (22%), lip

The Council of Europe appoints future EDQM Director
The Council of Europe has appointed Petra Dörr, PhD, as future Director of the European Directorate for the Quality of Medicines & HealthCare (EDQM). She will take over the function from the current Director, Susanne Keitel, in October 2021. Petra Dörr comes to the EDQM with more than 25 years of international experience in the public and private sector –
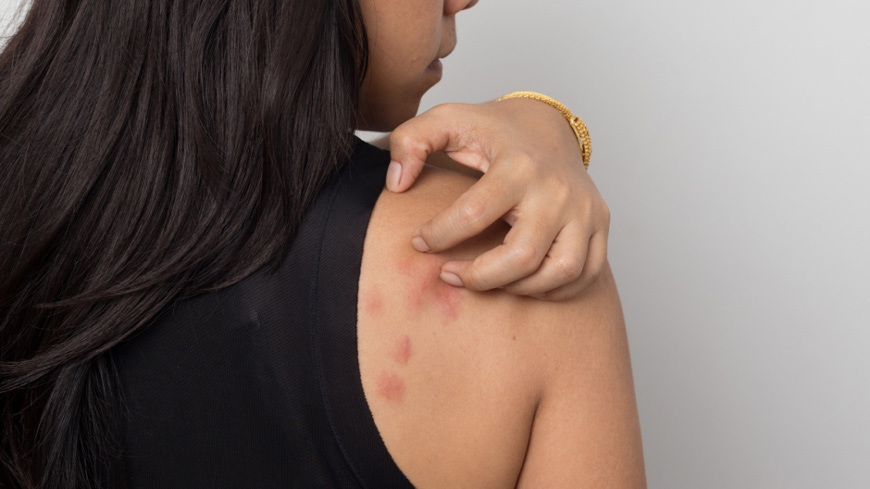
EDQM reports presence of allergenic fragrances in cosmetics sold as “perfume-free”
A study by the European Network of Official Cosmetics Control Laboratories (OCCLs) indicates that some cosmetic products sold throughout Europe still contain excessive levels of allergy-inducing fragrances. Results showed that 7.7% of samples were non-compliant with legislative requirements due to a missing or false declaration of allergenic fragrance compounds and that 3.1% of products marketed as “perfume-free” contained fragrance compounds.
Ten years at the service of consumer safety in Europe: over 600 analytical methods for cosmetics testing, 20 studies, 50 laboratories and more expected to join
The experts of the European Committee for Cosmetics and Consumer Health (CD-P-COS) and the members of the Network of Official Cosmetics Control Laboratories (OCCLs) held a virtual meeting on 22 and 23 June to define steps for advancing consumer protection in Europe and for reinforcing cross-border co-operation and independent cosmetics testing. The meeting also marked the 10th anniversary of the Network
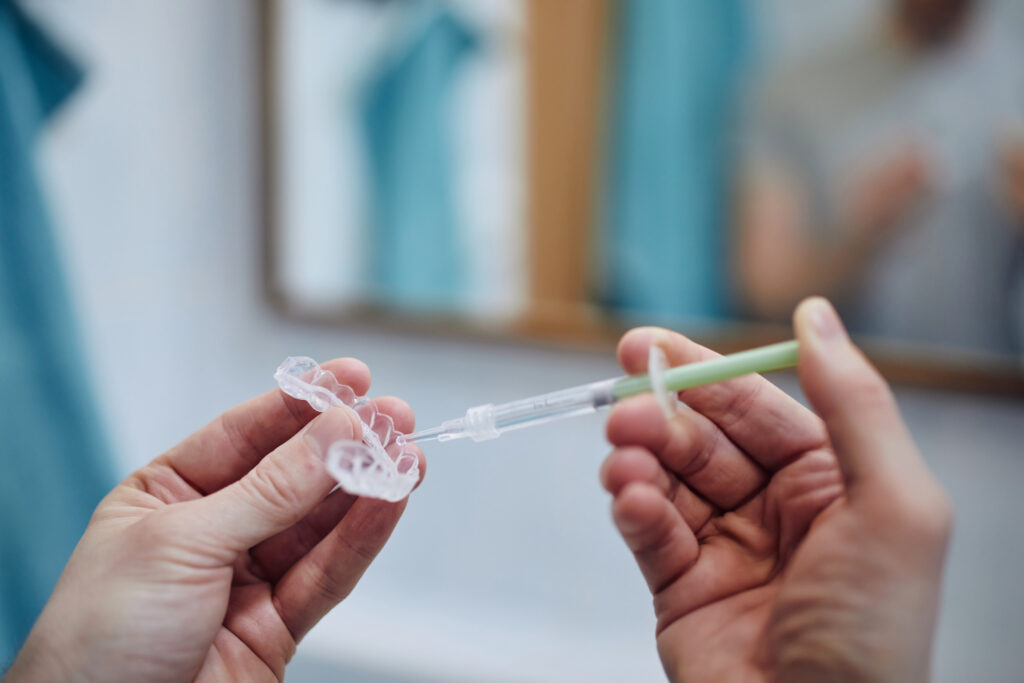
EDQM reports issues of non-compliance with tooth whitening products
The quality of tooth whiteners and whitening strips was assessed in a Market Surveillance Study carried out in 12 European countries and completed in 2018 by the network of Official Cosmetics Control Laboratories (OCCLs), coordinated by the EDQM. The findings, based on the testing of 261 samples of tooth whitening products, indicated that a large number of products were non-compliant
