Methods
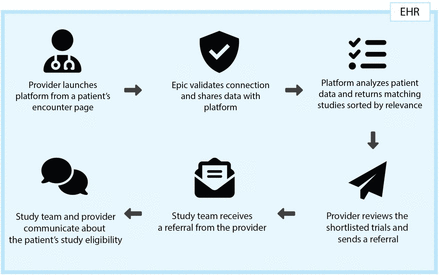
We customised a cloud-based platform (XpertScreen, developed by XpertDox, Birmingham, Alabama, USA), to streamline patient matching and study team referrals to ongoing clinical trials at cancer centres. This personalised trial matching system integrates with the EMR (Epic Systems, Verona, Wisconsin, USA) by passing through patient-associated data such as age, sex and primary oncologic diagnosis, as well as user authentication information, through a Substitutable Medical Applications, Reusable Technologies (SMART) on Fast Healthcare Interoperability Resources (FHIR) connection. It is instantly accessible within the patient EMR system for oncologists and other healthcare providers to initiate a clinical trial search (figure 1).
Workflow diagram of clinical trial screening. EHR, electronic health record.
At its core, XpertScreen leverages machine learning to navigate the complexity of trial eligibility criteria from ClinicalTrials.gov and internal datasets, transforming dense medical language into clear, actionable insights. Instead of requiring patients and clinicians to manually interpret intricate trial requirements, XpertScreen organises them into intuitive ‘Conditions’ and ‘Filters’ using a sophisticated NLP framework.
How it works
XpertScreen employs a multistage NLP pipeline to extract, classify and structure eligibility criteria, ensuring a precise and user-friendly experience (figure 2):
-
Named entity recognition: It identifies critical clinical entities such as age range, biomarkers, prior treatments and lab values using medical ontologies like Systematised Nomenclature of Medicine (a standardised clinical terminology), Unified Medical Language System (a framework for integrating biomedical terminologies) and Common Terminology Criteria for Adverse Events (a standardised classification for adverse effects in clinical trials).
-
Rule-based pattern matching: It detects numerical constraints (eg, age ranges) and captures key performance scores, lab values and treatment histories.
-
Ontology mapping and JSON structuring: It converts extracted data into a predefined JSON (JavaScript Object Notation, a lightweight data format for structured information) schema, standardising trial eligibility details across multiple data sources.
Illustrating the process behind how the clinical trial matching software works.
For example, an unstructured eligibility statement—‘Has previously failed all available and suitable therapies for AML. Disease relapse or the presence of refractory disease after ≥2 cycles of chemotherapy must be documented.’—is transformed into a structured JSON format, enabling seamless filtering and retrieval (online supplemental figure 1A).
Overcoming integration challenges
Achieving this level of automation and efficiency was not straightforward. Integrating with Epic’s electronic health record (EHR) system via SMART on FHIR (a standardised Application Programming Interface (API) framework for secure access to healthcare data) required careful mapping of structured patient data to the often unstructured criteria found in clinical trials. Handling authentication, patient consent and real-time queries—while ensuring strict Health Insurance Portability and Accountability Act (HIPAA) compliance—demanded meticulous engineering and rigorous testing.
Even though FHIR delivers EHR data in a structured JSON format, direct interpretation remains a challenge. Patient conditions, for instance, are often buried in deeply nested JSON objects with multiple identifiers (online supplemental figure 1B).
To ensure accurate filtering, we developed mappings for International Classification of Diseases (ICD) codes that unify related diagnoses, such as recognising both ‘Colon Cancer’ and ‘Colorectal Neoplasm’ as the same condition for clinical searches.
Beyond EHR integration, XpertScreen also syncs with OnCore, Stanford’s Clinical Trial Management System. OnCore plays a vital role in managing protocols, tracking participants, handling financials and ensuring regulatory compliance. To keep trial information accurate and up to date, we built a robust data pipeline that automatically ingests, standardises and updates trial details. This prevents outdated eligibility criteria from affecting the matching process, ensuring patients receive the most relevant and current options.
Streamlining access with security and oversight
To make trial access seamless for healthcare professionals, XpertScreen integrates with Stanford’s Single Sign-On, allowing clinicians, research coordinators and administrative staff to log in securely with their Stanford ID. This eliminates the need for additional credentials while maintaining high security and ease of use.
While automation plays a crucial role, human oversight remains essential. Each trial’s eligibility criteria must be carefully reviewed and validated through ongoing collaboration with PIs, research coordinators and regulatory teams. Because eligibility language varies across specialties and institutions, striking the right balance between automation and expert curation is key to maintaining accuracy and reliability.
Precision matching for better outcomes
XpertScreen categorises clinical trials based on key factors, ensuring precise patient-trial matching:
-
Cancer type: It matches patients to trials based on specific diagnoses (eg, breast, lung or other cancers).
-
Cancer stage: It aligns trials with disease progression, from localised to metastatic.
-
Surgical operability: It distinguishes trials for resectable and non-resectable tumours.
-
Therapeutic history: It considers prior treatments to determine eligibility.
-
Tumour-associated mutations: It identifies trials targeting specific genetic markers.
By bridging technical, operational and clinical gaps, XpertScreen is redefining how clinical trials are matched to patients, accelerating the journey from diagnosis to treatment. This innovation brings precision oncology closer to reality, empowering patients with access to cutting-edge therapeutic opportunities tailored to their unique medical profiles.
In practice
Once activated by a provider in the EMR, the matching system automatically imports comprehensive patient data, including demographic information and cancer stage, type and mutations. The user is prompted to verify and potentially edit the patient’s information, as needed. Using smart prompts, the user can enter such information in less than 15 s. The system stores patient’s information thereafter for future search, further improving the user experience. After the patient’s information is finalised, the system creates a rank-ordered list of potential trial options at the site, starting with the best match at the top, and always including at least one trial (figure 3A). From the list provided, the provider can select one or more eligible trial options for the patient and click the ‘Request Screening’ button to initiate a private health information (PHI)-secured email communication thread connecting the referring provider with the primary clinical research coordinators (CRCs) and PI (figure 3B). From a workflow perspective, CRCs and PIs have been requested to prescreen the patient within 48 hours based on verifying all information in the patient’s records in the EHR. If the patient is found to be eligible based on prescreening, the research team proceeds with consent and formal trial screening. The matching system strictly adheres to privacy standards, ensuring PHI/HIPAA compliance, SOC2 Type 2 certification, ISO:27001 certification and robust encryption for data both at rest and in transit.
(A) The matching system is integrated within the right-hand pane of the EMR. (B) The matching system screening request form uses secure email communication. EMR, electronic medical record.
All information on the matching system is updated two times a week, including updating the number of trials open or closed status and CRC contact information, thus minimising erroneous matches. Additionally, the matching system features an administrative console that grants the Clinical Trials Office administration the ability to manually adjust Conditions and Filters criteria—a feedback system through the matching system allows requests to be sent and tracked. Furthermore, the matching system records user logins, screening requests and, in the future, will allow follow-up of screening completion and assessment of platform utilisation.